Efficient conversion of CO2 is strategically significant for
alleviating the energy crisis and achieving the goal of carbon
neutrality. One promising conversion route is the hydrogenation of CO2 to methanol using a renewable energy-based "green hydrogen" source.
Traditional metal oxide catalysts for this reaction typically require a high temperature (>300 oC), which tends to promote undesired reverse water-gas shift (RWGS) side reactions, thus producing a large amount of CO as the by-product.
Introduction of transition metal components onto metal oxides can promote the activation of H2, thereby reducing the reaction temperature, but this also facilitates excessive hydrogenation of CO2 to CH4, leading to lowered methanol selectivity. Further improvement of the performance of conventional metal/metal oxide catalysts for low-temperature CO2 hydrogenation to methanol is severely restricted by the tradeoff between their activity and selectivity.
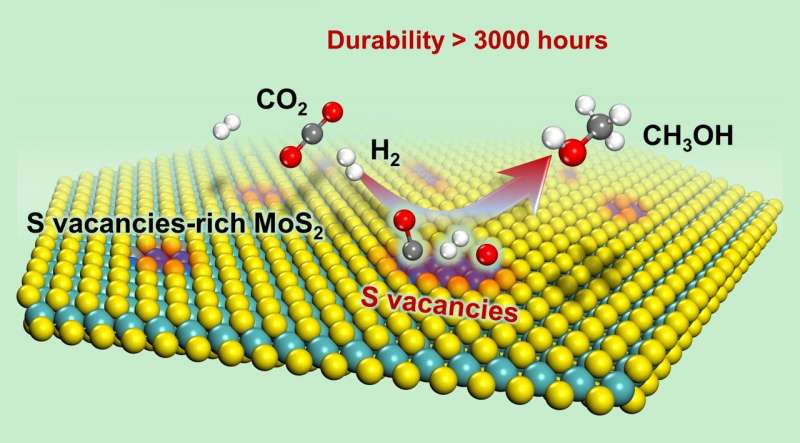
Recently, a group led by Prof. Deng Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Wang Ye from Xiamen University, achieved for the first time low-temperature high-efficiency hydrogenation of CO2 to methanol, with a long working life over sulfur vacancy-rich few-layered MoS2, as well as remarkably higher activity and selectivity than those of the commercial Cu/ZnO/Al2O3 catalyst.
Their work which was published in Nature Catalysis, opens up a new way for the conversion of CO2 with low energy consumption and high efficiency.
They found that the sulfur vacancy-rich few-layered MoS2 could simultaneously activate and dissoCiate CO2 and H2 at low temperatures and even at room temperature, thereby facilitating the low-temperature hydrogenation of CO2 to methanol with high activity and selectivity.
In addition, they found that the RWGS reaction and excessive hydrogenation of methanol to CH4 were effectively suppressed. At 180 oC, 94.3% methanol selectivity for a CO2 conversion of 12.5% was achieved over the catalyst; this result was better than that obtained with the commercial Cu/ZnO/Al2O3 catalyst and previously reported catalysts.
The activity and selectivity were steadily maintained for over 3000 hours over the MoS2 catalyst, rendering it a promising candidate for industrial applications. In situ characterizations combined with theoretical calculations demonstrated that the in-plane sulfur vacancies on MoS2 were the active centers for catalyzing the highly selective hydrogenation of CO2 to methanol.
"This work reveals the potential of in-plane vacancies in
two-dimensional materials for catalysis and provides a novel strategy
for the development of new catalysts to be used in CO2 hydrogenation" said Prof. Deng.

