As the planet's burden of rubber and plastic trash rises unabated, scientists increasingly look to the promise of closed-loop recycling to reduce waste. A team of researchers at Princeton's Department of Chemistry announces the discovery of a new polybutadiene molecule—from a material known for over a century and used to make common products like tires and shoes—that could one day advance this goal through depolymerization.
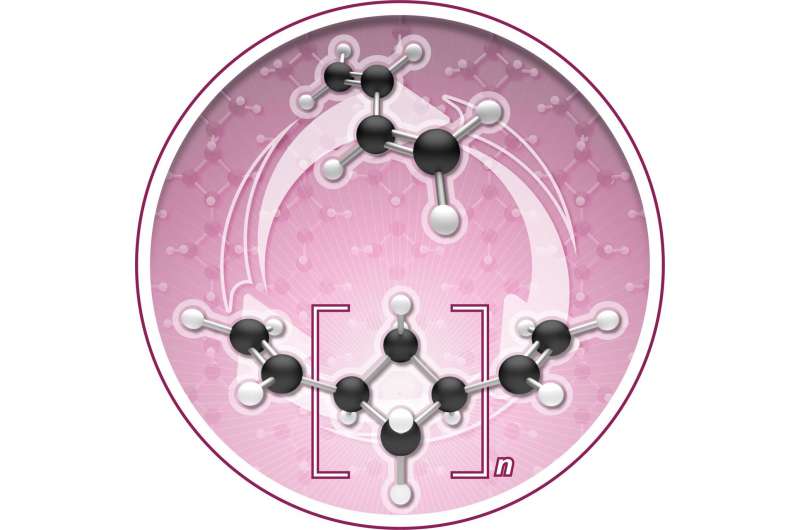
The Chirik lab reports in Nature Chemistry that during polymerization the molecule, named (1,n'-divinyl)oligocyclobutane, enchains in a repeating sequence of squares, a previously unrealized microstructure that enables the process to go backwards, or depolymerize, under certain conditions.
In other words, the butadiene can be "zipped up" to make a new polymer; that polymer can then be unzipped back to a pristine monomer to be re-used.
The research is still at an early stage and the material's performance attributes have yet to be thoroughly explored. But the Chirik lab has provided a conceptual precedent for a chemical transformation not generally thought practical for certain commodity materials.
In the past, depolymerization has been accomplished with expensive niche or specialized polymers and only after a multitude of steps, but never from a raw material as common as that used to make polybutadiene, one of the top seven primary petrochemicals in the world. Butadiene is an abundant organic compound and a major byproduct of fossil fuel development. It is used to make synthetic rubber and plastic products.
"To take a really common chemical that people have been studying and polymerizing for many decades and make a fundamentally new material out of it—let alone have that material have interesting innate properties—not only is that unexpected, it's really a big step forward. You wouldn't necessarily expect there still to be fruit on that tree," said Alex E. Carpenter, a staff chemist with ExxonMobil Chemical, a collaborator on the research.
"The focus of this collaboration for us has been on developing new materials that benefit society by focusing on some new molecules that [Princeton chemist] Paul Chirk has discovered that are pretty transformative," Carpenter added.
"Humankind is good at making butadiene. It's very nice when you can find other useful applications for this molecule, because we have plenty of it."
Catalysis with Iron
The Chirik lab explores sustainable chemistry by investigating the use of iron—another abundant natural material—as a catalyst to synthesize new molecules. In this particular research, the iron catalyst clicks the butadiene monomers together to make oligocyclobutane. But it does so in a highly unusual square structural motif. Normally, enchainment occurs with an S-shaped structure that is often described as looking like spaghetti.

