Biosensors measure the concentration of molecules in biological samples for biomedical, environmental, and industrial applications, and, ideally, they should provide real time, continuous data. However, the continuous monitoring of small molecules at low concentrations is problematic. Researchers at Eindhoven University of Technology have developed an innovative sensing approach based on molecular lookalikes. This could prove pivotal in future biosensors for monitoring health and disease.
The field of biosensors has a rich and inventive history. The "father of biosensors" is seen by many to be Leland C. Clark Jr., who designed a sensor to measure oxygen in blood in the early 1960s.
However, as happens in pioneering works, things didn't start out as he had hoped. His initial sensor designs failed because blood components affected the sensing electrode.
Clark's solution was to separate the electrode and the blood by a cellophane wrapper from a cigarette packet, which proved to be the solution needed to reliably measure oxygen in blood. A prime example of being creative and innovative in the laboratory!
Fast-forward to 2020, and researchers from the Departments of Biomedical Engineering and Applied Physics at TU/e are demonstrating similar inventiveness when it comes to sensing low-mass molecules of interest.
In a paper published in ACS Sensors, Junhong Yan, Menno Prins, and colleagues showcase a new approach that can continually measure the concentration of low-mass molecules of interest in biological samples based on biosensing by particle mobility (BPM).
"Our approach is a platform for future biosensors to continually monitor markers associated with personal health conditions such as kidney or liver failure," says Yan.
Biosensors 101
Existing biosensors typically give a single measurement result from a single biological sample. The sample can be blood, sweat, urine, or saliva, and the result can be the level of a protein, a hormone, a drug, or a virus in the sample.
However, it would be better if sensors give a continuous stream of data rather than only a single data point, because that would allow an individual to monitor how a medical condition develops over time.
The only continuous biosensor that is presently commercially available is the Continuous Glucose Monitor (CGM) that continuously measures glucose in interstitial skin fluid, which is very useful for individuals with diabetes. Unfortunately, molecules other than glucose cannot yet be measured continuously. This presents a significant opportunity for sensor innovation!
Every biosensor consist of three main parts—a molecular component that involves a bioreceptor that can bind to the molecule of interest, a transducing principle that converts the molecular recognition into a detectable signal, and a detection system that records the signal and presents the answer as a number, graph, sound, or light indication to be easily interpreted by the user.
"In this work we have focused on the first part—devising a molecular principle to continuously measure molecules of interest with low molecular mass and low concentration," says Prins.
Molecular lookalikes
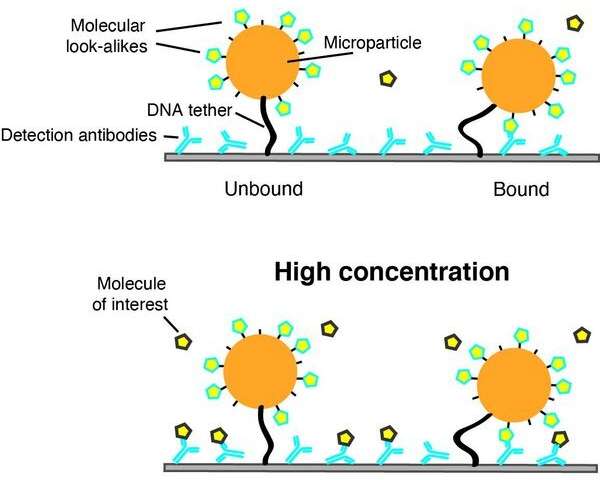
The sensor designed by Yan, Prins, and the team adopted the use of molecular lookalikes or fake versions of the molecules of interest.
So how do these lookalike molecules assist in the detection of the real molecules? Menno Prins explains more: "The surface of the sensor is coated with antibodies that can bind to the molecules of interest. When there are no molecules in the test fluid, the lookalike molecules are free to bind to the antibodies. However, when there are molecules of interest in the fluid, these can bind to the antibodies. As a result, the lookalikes are released from their binding to the antibodies."
The lookalike molecules do not freely move around the sensor like the molecules of interest do in a test fluid. These lookalike molecules are attached to a microparticle, which is tethered to the surface of the sensor using DNA so that switching between bound and unbound states can be detected.
Binding is the key
The operation of the sensing platform is quite simple, and brilliant it must be said. All molecular binding events are designed to be reversible. This includes the binding between antibodies and the lookalikes, and the binding between the antibodies and the molecules of interest in solution.
Repeated binding and unbinding events involving the lookalike molecules or the molecules of interest in a fluid take place, and these events can easily be measured using optical microscopy by recording the state of the microparticle.
When there is a high concentration of the molecules of interest in a solution, then most of the antibodies on the sensor surface are blocked. This reduces the potential for the microparticles to switch to a bound state. On the other hand, when the concentration is low, then many switches occur between bound and unbound states due to the reversible bindings of the molecular lookalikes.
"Detection of binding and unbinding events of a large number of particles caused by the specific molecular interactions is key to the technology, allowing us to measure small changes of molecular concentration in the fluid," says Yan.
Passing tests and next steps
To test their new approach, the authors designed sensors to monitor the concentrations of short single-stranded DNA fragments and of creatinine. The concentrations were monitored over hours, with a time resolution of a few minutes.
Creatinine is a metabolite molecule with a small mass of only 113 Dalton that is a marker for kidney function. The marker could be measured in the medically relevant range between 10 μM and 10 mM. Single-stranded DNA could be measured between 10 nM and 1 μM.
"These results are very promising and demonstrate that small molecules can be continuously monitored across a wide range of concentrations. Our next aim is to demonstrate the technology for a wide variety of molecules and biological fluids, for enabling future applications in healthcare, and in industrial process and environmental monitoring" says Prins.
This innovative sensing approach may very well solve issues with the detection of low-molecular mass biomarkers for our future biosensor needs.
While the approach is a little more sophisticated than the use of cellophane wrapper on an electrode, it's quite likely that the late Leland C. Clark Jr. would have been impressed.

