Electrocatalysis is one of the most studied topics in the field of
material science, because it is extensively involved in many important
energy-related processes, such as the oxygen reduction reaction (ORR)
for fuel cells, the hydrogen evolution reaction (HER) for green hydrogen
production, and the oxygen evolution reaction (OER) for metal-air
batteries. Noble metal aerogels (NMAs) emerge as a new class of
outstanding electrocatalyst due to the combined feature of metals and
aerogels. However, limited by the available compositions, the explored
electrocatalytic reactions on NMAs are highly restricted and certain
important electrochemical processes have not been investigated.
Alexander von Humboldt research fellow Ran Du in collaboration with Prof. Wei Jin from the Jiangnan University, China, recently created various noble metal aerogels, disclosing their unprecedented potential for diverse pH-universal electrochemical catalysis, including ORR, HER, and electrochemical water splitting. These findings largely span the application territory of NMAs for fuel cells, green hydrogen production and many more. The work was published in the renowned journal Advanced Energy Materials.
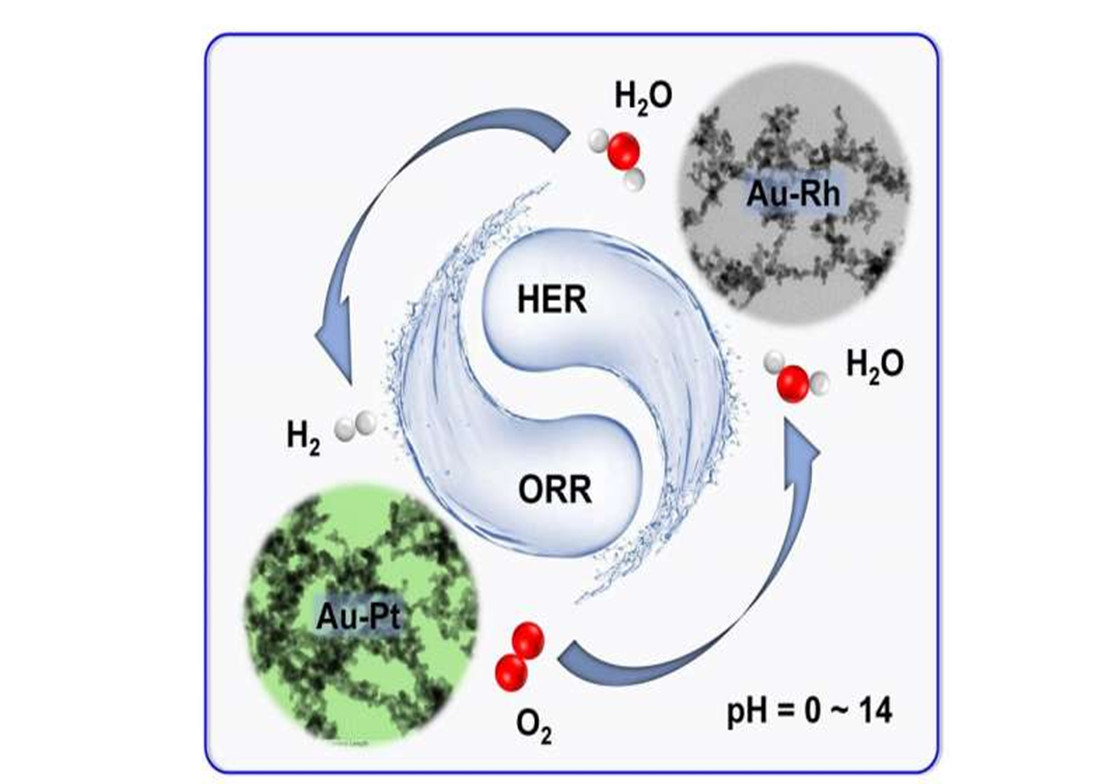
By adopting a strong salting-out agent (i.e. ammonium fluoride [NH4F])
as an initiator to trigger gelation, the composition of the as-obtained
noble metal aerogels (NMAs) was extended to various bi- and trimetallic
systems. Subsequently Ran Du and his team manipulated the chemical composition,
thus expanding the application territory of NMAs to pH-universal ORR
electrocatalysis, HER electrocatalysis, and electrochemical water
splitting. Notably, the Au-Rh aerogel and Au-Pt aerogel manifested
extraordinary pH-universal performance for HER and ORR electrocatalysis,
respectively, both of which considerably outperform commercial Pt/C
(platinum on carbon) in wide pH environments.
"Further research directions may be placed on morphology-controlled NMA synthesis, and the establishment of the correlations between the structural features of NMAs and their electrocatalytic properties," assumes chemist Ran Du.

