Carbohydrates are complex molecules, difficult to synthesize in the lab, but doing so is useful in studying beneficial sugars such as those found in human breast milk, or enabling researchers to tailor the chemical structure of drug candidates, vaccines and natural products.
University of Michigan chemists have developed a simple method of synthesizing carbohydrates that widens the range of labs that can use synthetic chemistry to generate and study novel carbohydrate structures. Their results are published in the Journal of the American Chemical Society.
"Nucleic acids, proteins, and carbohydrates are three of the basic building blocks you learn about in your biology classes," said lead author John Montgomery, professor of chemistry and medicinal chemistry. "Chemistry has been able to automate the preparation of nucleic acids and proteins to where accessing these structures is routine, but carbohydrates are orders of magnitude more difficult to prepare. There are some incredible advances being made in the automation of carbohydrate synthesis, but the fact remains that this is tough chemistry that is holding back advances in glycobiology."
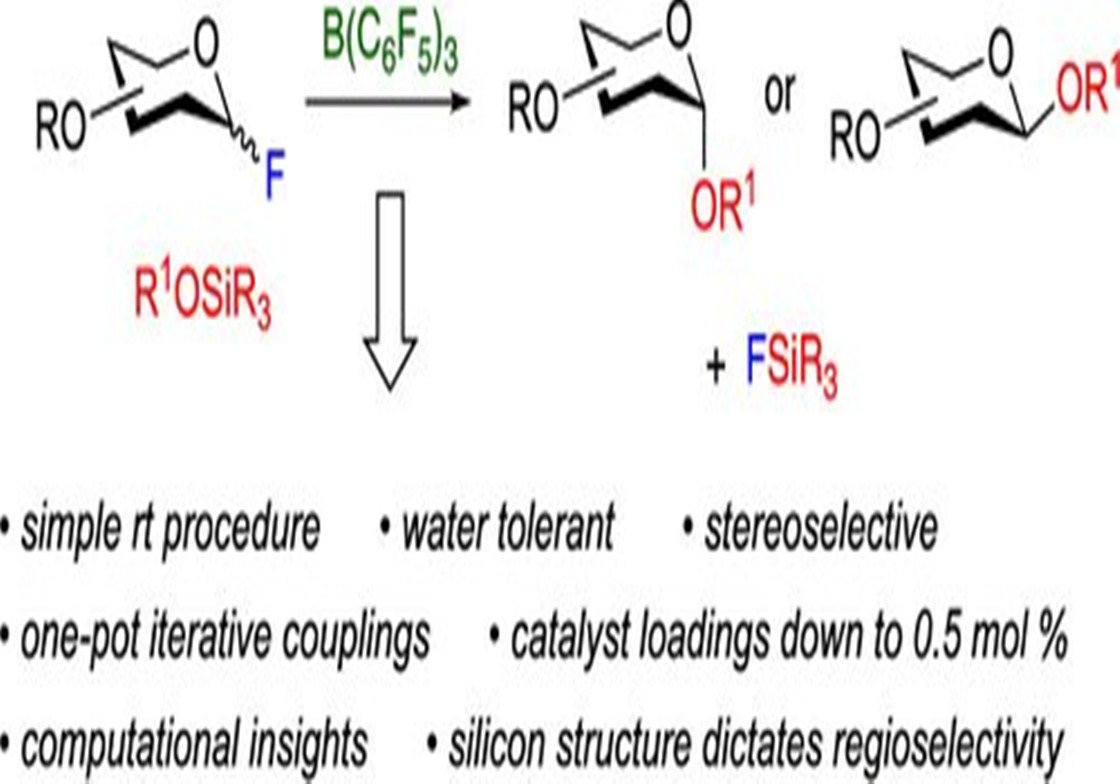
Carbohydrates have great structural diversity, complex branching patterns, and 3-D architecture that hinder scientists' ability to synthesize the compounds, says Montgomery. By using a silicon group to pre-engineer how carbohydrates will react with each other, the U-M team's method is able to control the carbohydrate's branching pattern and reduce the steps required to access more complex structures.
For example, carbohydrate molecules typically consist of carbon, hydrogen, oxygen and sometimes nitrogen atoms and range in length, with five- and six-carbon sugars being most prevalent. Most of the carbon atoms have an alcohol group, which can be connected to the next carbohydrate in a myriad of possible patterns.
"This branching feature is what makes synthetic chemistry very tedious because you have to control the selectivity between what part of the molecule is going to react with the next," Montgomery said.
To control how those alcohol groups click carbohydrates together, Montgomery and his team put silicon "protecting groups" on select alcohols within a carbohydrate.
"Normally protecting groups need to be taken on and off during the synthesis, adding time and cost to the procedure. Our strategy is designed so that the protecting group naturally falls off during the coupling reaction and times the sequence of which alcohol group reacts when desired," Montgomery said. "By this approach, we can reverse the reactivity of two alcohols, or we can take two alcohols that would normally have similar reactivity and make one react selectively over the other without additional steps."

