In a new study published on Science Advances, Yichao Zhao and a research team in integrated bioelectronics and materials and engineering in the U.S. engineered a disposable, free-standing electrochemical sensing system (FESS). The FESS allowed them to realize a system-level design strategy to address the challenges of wearable biosensors in the presence of motion and allow seamless integration with consumer electronics. The team developed a FESS-enabled smartwatch, featuring sweat sampling, electrochemical sensing and data display or transmission, within a self-contained wearable platform. The team used the FESS-smartwatch to monitor the profiles of sweat metabolites among individuals in sedentary and high-intensive exercise settings.
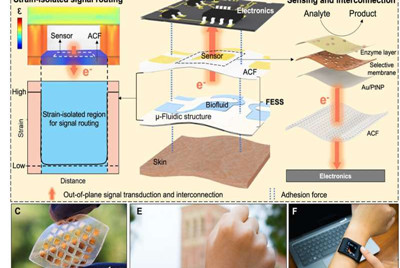
The internet-of-things (IOT) infrastructure can be used in wearable consumer electronics to transform personalized and precision medicine by harvesting physiologically relevant data with minimal user intervention. Scientists have typically used physical sensors in commercial wearable platforms to track a user's physical activity and vital signs. However, to gain insight into the body's dynamic chemistry, researchers require electrochemical sensing surfaces to target the biomarker molecules within non-invasively retrieved body fluids such as sweat. To accomplish this, it is critical to precisely engineer the information delivery pathway from the skin to a readout unit. For electrochemical sensing, the information delivery pathway must sample and deliver the biomarker-rich biofluid to the sensor surface in a microfluidic structure, followed by signal transduction through interconnected elements to the readout electronics. The signal must be maintained along this pathway in the presence of motion-induced strain.
In this work, Zhao et al. developed the freestanding electrochemical
sensing system (FESS) and simultaneously adhered it to the skin and to
electronics using double-sided adhesion forces without rigid connectors.
The FESS sampled and directed epidermally
retrieved biofluids for electrochemical sensing, followed by routing to
readout electronics through a strain-isolated pathway. They integrated
the FESS inside a custom-built smartwatch for sweat induction, sampling,
electrochemical sensing, signal processing and data display or
transmission. The results showed high-fidelity signal transduction and
robust mechanical contact with human skin without constraining user
motion. The freestanding sensing system can be linked with future
wearable electronics to generate high-fidelity health and
wellness-related datasets based on the daily activities of users.
To create an efficient biological pathway, Zhao et al. selected integrin—a cell adhesive molecule that efficiently allowed physiological information exchange between intracellular and extracellular matrices. The FESS device implemented integrin-like functionalities through a strain-isolated region in a microfluidic structure. They engineered FESS as a vertically conductive, double-sided adhesive and flexible microfluidic bioanalytical thin film system composed of multiple vertically stacked films. These films included an adhesive anisotropic conductive film (ACF), a noble metal electrode array film, a biochemical film, a microfluidic film and a skin adhesive film. They taped the complete thin film system onto the readout electronics without connectors and with minimal contact resistance to potentially transform any electrical contact into a chemical or biological sensor. The team developed a proof-of-principle, self-contained biomarker-sensing smartwatch with FESS to monitor the sweat metabolite profiles of individuals in sedentary vs. high-exercise settings.
In this setup, the vertical conductivity of ACF facilitated out-of-plane signal interconnections to avoid undesired body-motion-induced strain effects on the signal pathways. The team characterized the mechanical adhesion property of FESS to ensure the adhesion forces between the FESS and electronics were higher than those between FESS and dry or actively sweating skin. The team tested the force required to peel the ACF layer from the FESS on a printed circuit board and the results showed a strong FESS-based interconnection to electronics, as suited for on-body applications.
Zhao et al. then tested the signal transduction capability of the FESS. They patterned noble metal electrodes onto the ACF to achieve biochemical-to-electrical signal transduction, followed by deposition of biochemical films to analyze biomolecular targets of interest. They tested the electrochemical activity of metal-patterned ACF for two commonly used electrode surfaces on unmodified gold (Au) and platinum (Pt) nanoparticle-modified Au. The electroanalytical methods investigated in the work provided sample-to-answer biomarker readouts to obtain real-time insight into the alterations in sweat biocomposition.
In the next few experiments, the research team showed the capability of FESS to monitor biomarkers during the user's daily activities. To accomplish this, they integrated FESS into a custom-developed smartwatch as a model IOT device containing and analog/digital circuit, Bluetooth transceiver and a liquid crystal display (LCD) screen for system-level functionalities, including signal and user-command processing, display and wireless data communication. The FESS-based smartwatch performed similarly to a potentiostat. The scientists adhered the complete smartwatch onto the skin without external wrappings or fixtures for wireless biomarker sensing as a self-contained unit. The LCD screen displayed real-time readings and temporal profile of the biomarker measurements, while the Bluetooth receiver relayed the readings to a custom-developed mobile application to upload the data to a cloud-server for further analysis.
The team adhered the FESS-based smartwatch onto a subject's forearm to display its function as a wearable system to monitor biomarkers. The subject could wirelessly control the device to take real-time, sweat-based biomarker measurements relative to the user's daily routine. The user monitored their sweat glucose levels before or after consuming a mixed range of meals and the readout of the smartwatch indicated elevated sweat glucose levels after food intake, in alignment to previous trends. The smartwatch additionally provided user information on sweat lactate readings while running in a field, the results were consistent despite the involvement of high-frequency and high-acceleration-based body motions.
In this way, Yichao Zhao and colleagues examined the biomarker
information delivery pathway and recognized near-zero strained regions
inside a microfluidic-sensing module to engineer a strain-isolated path
to preserve the fidelity of biomarker data. The thin-film system that
formed the free-standing FESS entity was bioinspired by integrin-like
functionalities for signal transduction
and signal interconnection via double-sided adhesion. The FESS
efficiently bridged the skin and readout electronics to harvest
biomarker information. The team coupled the FESS system seamlessly with a
custom-developed smartwatch as a wearable biosensor to monitor
real-time biomarker readouts throughout a user's daily routine. To
commercialize the prototype developed in this work, Zhao et al. propose
future clinical trials to map sweat-based biomarker readings and gain
information on the physiological status of the users. The advantages of
this work, including their ease of integration with wearable electronics
and high fidelity readings can be employed to perform large-scale
clinical investigations.

