Electrocatalysis is extensively involved in many important energy-related processes such as the oxygen reduction reaction (ORR) for fuel cells, the hydrogen evolution reaction (HER) for green hydrogen production, and the oxygen evolution reaction (OER) for metal-air batteries. Noble metal aerogels (NMAs) have emerged as a new class of outstanding electrocatalysts, combining the features of metals and aerogels. However, the development of these porous materials has been impeded by sluggish fabrication methods, which require several hours or even weeks. In addition, the unique optical properties of noble metals—for instance, plasmonic resonance—have so far been ignored in NMAs, limiting their potential high performance in electrocatalysis.
Ran Du from China is an Alexander von Humboldt research fellow who has worked since 2017 as postdoc in the physical chemistry group of Professor Alexander Eychmüller at TU Dresden. Together, they recently revealed an unconventional self-healing behavior in noble metal gels, a rare characteristic in solely inorganic gel systems. On this basis, they developed a method for tremendously accelerating the gelation speed. Their findings were published in the journal Matter.
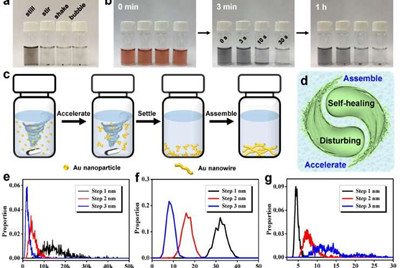
This unconventional and conceptually new strategy for rapid gelation is a counterintuitive disturbance-promoted gelation method. The in situ introduction of a disturbance field during the gelation greatly facilitates mass transportation and induces accelerated reaction kinetics. Upon removal of the disturbance field, the resulting gel pieces can reassemble to a monolith via the self-healing property. This leads to gelation within one to 10 minutes at room temperature without affecting the microstructures of gels. This is two to three orders of the magnitude quicker than traditional approaches. The mechanism was also supported by Monte Carlo simulations. Notably, the disturbance methods can be expanded to shaking and bubbling, and the method is applicable to various compositions, such as gold (Au), palladium (Pd), rhodium (Rh), gold-palladium (Au-Pd), gold-palladium-platinum (Au-Pd-Pt), and morphologies, for example, the core-shell structure or homogeneous structure.
Ran Du also took advantage of the combined optic and catalytic activities of noble metals: "We also were first to demonstrate the photoelectrocatalytic properties of NMAs by using ethanol oxidation reaction (EOR) as a model reaction, displaying an activity increase of up to 45.5% by illumination and realizing a current density of up to 7.3 times higher than that of commercial palladium/carbon (Pd/C). Thus, we pioneered the exploration of photoelectrocatalysis on NMAs, opening up new space for both fundamental and application-orientated studies for noble metal gels and other systems."

