Researchers here have come up with a strategy to make a transition metal-catalysed chemical reaction enantioselective through the use of a chiral cation. The work was carried out in Dr. Robert Phipps's research group.
Previously, Robert says, while chiral cations have been widely used as organocatalysts, bringing them into chemical reactions catalysed by transition metals to control the enantioselectivity of the reaction has been much rarer. Now, following two years' hard work "and a lot of team effort," they have succeeded in doing so.
Their work, published in Science, is "essentially a proof-of-concept that you can do this, and that it can be done on a challenging reaction type that has proven tough with existing approaches," he says.
The Phipps group develops methodologies for the synthesis of small molecules, such as drugs. In the last few years, they have devoted significant effort to developing catalysts that are able to control the positional selectivity in chemical reactions—i.e. controlling at exactly which point on the molecule the chemical reaction takes place. They have done this by developing ligands for transition metal catalysts that anchor the catalyst to a particular position on the molecule.
And they have been looking for ways to refine the ligands even further, so that they are able to make them not just positionally selective but enantioselective as well. Enantiomers are mirror images—like a left hand and a right hand—of a molecule. Being able to select which of the two enantiomers of the molecule they produce, and therefore which type of 'handedness' it has, is key.
For while one enantiomer of the molecule may produce the desired therapeutic effect, the other enantiomer may either produce no effect at all, or may bring unwanted side effects. Additionally, the chirality, or 'handedness," of the enantiomer can be vital in ensuring precision in how it interacts within a biological system.
"Nature itself exhibits chirality in multiple ways. Proteins are formed of amino acid building blocks that exist naturally as one enantiomer, i.e. they are one-handed. Plus DNA possesses helical chirality," Robert explains. "So as synthetic chemists, it's really important that we are able to make small molecules as single enantiomers. When we are going to put a small molecule into a biological system where we hope it will have, for example, a therapeutic effect, the 'left-hand' enantiomer might fit in quite differently when compared to the 'right-hand' enantiomer."
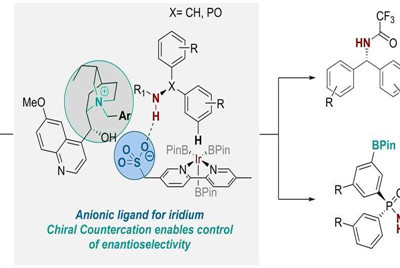
Three years ago, the group developed a ligand for transition metal catalysts that controlled the positional selectivity in a C-H activation reaction—a useful reaction that's widely employed in the pharmaceutical industry and is usually achieved using transition metal catalysis. They did this through taking a common bipyridine ligand scaffold and attaching an anionic sulfonate group to it.
Having attached this anionic (or negatively-charged) group to the scaffold, they then needed a positively-charged component to balance it. In their previous work, the researchers used a run-of-the-mill cation (tetrabutylammonium) for this role, which was initially just to provide solubility of the salt in organic solvents. But they realised that "we had an opportunity to bring in a chiral cation here that could potentially be involved in the transition state of the reaction and exert some influence on it," Robert explains. So they set out to explore whether this was possible.
Ph.D. student Georgi Genov first worked on the idea, which ultimately involved two further Ph.D. students, James Douthwaite and David Gibson, and a postdoctoral researcher, Antti Lahdenper?. They replaced the achiral tetrabutylammonium cation with a chiral cation derived from the naturally occurring anti-malarial quinine. And by doing so, they found they were able to control not only the positional selectivity but also the enantioselectivity in the reaction, which replaces the a C-H bond on an aromatic ring with a versatile C-B bond.
Georgi says: "The optimisation process for this transformation was quite involved. However, after discovering the right cation and reaction conditions, we observed that the system was remarkably general allowing us to utilise two very different substrate classes—one where the new stereocentre is at a carbon atom, and one where it is at phosphorus. The latter is very exciting as synthesis of chiral at phosphorus compounds is more challenging."
Hitherto, there have been very well established ways of doing enantioselective reactions with transition metals—but they normally rely on introducing some kind of chirality directly into the ligand scaffold attached to the metal and "there can be disadvantages to this approach which mean it won't work in some situations," Robert says.
"We know that these chiral cations, of the type we are using, are really privileged because they have been used in other types of asymmetric catalysis. But they have hardly been used at all with transition metals. So if we could potentially unite these privileged chiral cations with reactive transition metals, then maybe we've got a new and quite different way of thinking about doing asymmetric transition metal catalysis."
He adds: "We are excited to look at rolling out this to other really useful transition metal-catalysed reactions where challenges exist in performing them enantioselectively. Specifically, we'd like to see if we can enable enantioselective reactions that haven't been possible before, using this strategy."

