A chemist from RUDN University has proposed a new method for the synthesis of secondary propargylamine used to create antidepressants, drugs for Parkinson's disease and other neurodegenerative diseases, as well as malaria. It differs from the known methods in its simplicity and stability of the resulting substance. The paper was published in the Journal of Organic Chemistry.
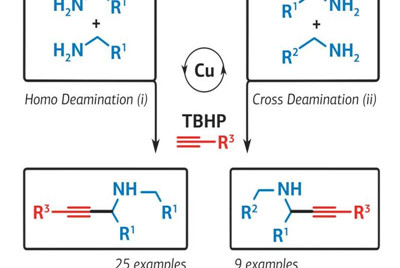
Propargylamines, which are the main active ingredients in many drugs for neurodegenerative diseases and malaria, are produced through a complex and energy-intensive process. This is due to the low reactivity of the starting components: primary amines and intermediate imines. In addition, with existing synthesis methods, the final product is excessively oxidised, which reduces the yield of the pure product to about 35 percent of what is theoretically possible. A chemist from RUDN University and his colleagues proposed a simpler method for the synthesis of propargylamines, which makes it possible to obtain an oxidation-resistant final product and increase the synthesis efficiency up to 56 percent.
"Propargylamines are widely used for the synthesis of organic compounds. In particular, they are used in medicine, since they exhibit antitumour activity and lower blood pressure. Propargylamines are part of anti-malaria drugs, medications for Parkinson's disease, and antidepressants. As a rule, they are synthesised from simpler substances, amines and alkynes, with the formation of an intermediate product, imine. The reaction is weak because the intermediate imine reacts with other substances weakly," says chemist Eric Van der Eycken from the Microwave-assisted Organic Synthesis research center of RUDN University.
The chemist suggested that the synthesis could be carried out through a cascade of chemical reactions of amines and alkynes using a copper-based catalyst. The first attempts did not result in the final product, since the copper catalysts (copper chloride, copper iodide, and copper bromide) were used and they showed low activity in this reaction.
Then the researcher changed the temperature, the type of solvent, and the ratio of the starting materials. As a result, optimal conditions and the most effective copper catalyst, copper acetylide, were chosen. The chemist did synthesize propargylamines, and the yield of the final product was 56 percent, which is significantly higher than the 35 percent possible with the traditional method of synthesis.
The chemist also found that the efficiency of the reaction and the yield of the final substance depend on electronic effects: a shift in the electron density in the molecule, ion, or radical under the influence of substituent atoms, which do not participate in the reaction directly, but affect the reacting part of the molecule. This phenomenon should be taken into account when designing a synthesis process to obtain the highest yield of a substance.
Thus, the chemists have developed a new method for production of propargylamines and their derivatives from amines and alkyne. The reaction will allow obtaining propargylamines at a lower cost and with a large yield, for their further use in organic synthesis and medicine, including the production of popular and essential drugs, as well as medications for neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and Lewy body dementia.

