A RUDN chemist and colleagues from Iran have developed a new strategy for producing hollow, porous catalysts for the Heck coupling reaction. These catalysts are characterized by a flower-like structure. They consist of graphene, on the surface of which layered aluminum-cobalt hydroxides and palladium nanoparticles are deposited. The resulting material reduces the synthesis time of the substance necessary to obtain dyes—trans-stilbene—by 10 times without reducing the yield of the target product. The developed catalysts can be applied in stereoselective synthesis in pharmaceuticals, the preparation of organic dyes, and in agrochemistry. The results were published in the journal Catalysis Letters.
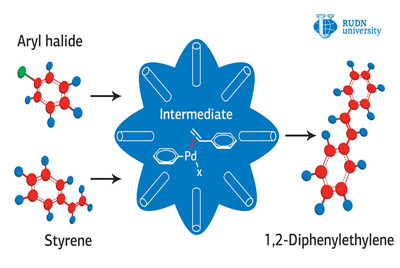
The Mizoroki-Heck reaction is used to create dyes, herbicides and medicines in which palladium acts as a catalyst. Catalysts are usually combined with double-layered aluminum and cobalt hydroxides to increase the catalytic activity of palladium. But low electrical conductivity, poor mechanical stability and short life cycle of these hydroxides limit their use. The chemists overcame these obstacles by introducing graphene oxide into the catalyst structure. Nitrogen and sulfur atoms on the surface of graphene increase the binding strength with palladium nanoparticles. Graphene can be an ideal carrier for palladium and cobalt and aluminum hydroxides due to its large surface area, chemical stability, and high electrical conductivity.
Rafael Luque, director of the Research Center for Molecular Design and Synthesis of Innovative Compounds for Medicine at RUDN University, and colleagues first oxidized graphene to oxide. Then, they synthesized flower-like hollow spheres in the presence of cobalt and aluminum salts. After that, they implanted sulfur and nitrogen atoms into them at high temperature and pressure. The chemists synthesized the target catalyst by recovering palladium compounds in the pores of the resulting hollow spheres. The catalytic activity was investigated by heating the catalyst with styrene and aromatic derivatives of chlorine, bromine and iodine.
"These catalysts are very efficient and have a very high stability as compared to other reported systems," says Luque.
The researchers revealed a high catalytic activity in the obtained porous spheres with the release of trans-stilbene—the target reaction product—up to 95 percent within two hours of the reaction under mild synthesis conditions. The authors also found that iodine derivatives react faster than similar bromine and chlorine derivatives. The catalyst showed the ability to react after eightfold repeated use, which indicates its high stability. Previous catalysts for the Heck coupling reaction either showed lower yield values from 62 percent to 91 percent, or required a high temperature and reaction time. Rafael Luque and his colleagues proved the potential of creating a flower-like stable palladium hollow catalysts and the positive role of graphene alloyed with sulfur and nitrogen on a substrate of layered cobalt aluminum oxides.

