Researchers at the Czech Academy of Sciences and the Brno-based start-up CF Plus Chemicals, a spin-off of ETH Zurich, has reported a new technology called CF LINK for site-selective bioconjugation of proteins and their structural characterization. The technology can selectively prepare protein conjugates via their tryptophan residues and perform post-translational modification of aromatic amino acids. Furthermore, it can also be used as a tool for the mapping of protein surfaces and studies of protein-protein interactions.
The company CF Plus Chemicals, an ETH Zurich spin-off founded in 2014, is based on almost 10 years of cooperation of the group of Dr. Petr Beier at the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences (IOCB Prague) and Dr. Václav Matou?ek, a Ph.D. alumnus of Prof. Dr. Antonio Togni at ETH Zurich.
Reagents based on cyclic hypervalent iodine-perfluoroalkyl compounds, also called Togni reagents, have become widely used and popular tools in organic synthesis, especially in medicinal chemistry for preparation of new fluorinated drug candidates, in line with the growing demand for fluorinated scaffolds in drug design. In the early 1990s, fluorinated molecules accounted for 5 percent of the total number of approved drugs; they now comprise 15 percent, and roughly 30 percent for newly approved therapeutics.
This family of chemical compounds was subsequently expanded in 2013 in collaboration of ETH Zurich and Dr. Petr Beier from IOCB to a new generation of patented Togni reagents that carry a more complex RCF2CF2 group instead of a simple CF3 group. The new family of these chemicals shares not only the rich reactivity of the first generation of Togni reagents, but also exhibit virtually unlimited structural variability of the β-substituted tetrafluoroethyl group, which they are able to transfer to a variety of substrates relevant to medical chemistry of small molecules.
In 2017, the application potential of the second generation of Togni reagents was extended to proteins. Their high affinity toward the thiol group allows selective bioconjugation through cysteines to form stable conjugates which, unlike maleimide conjugates, are not subject to slow deconjugation and thiol exchange.
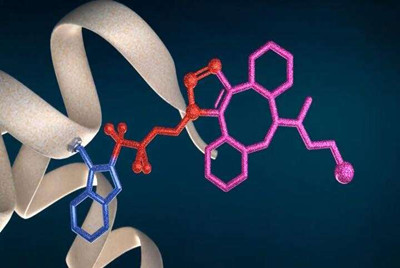
The present invention builds on the previous knowledge of the radical nature of Togni reagent-mediated reactions. In the SME Instrument Horizon 2020 project, supported by the City of Brno and in cooperation with IOCB and Dr. Petr Novák from the Institute of Microbiology of the Czech Academy of Sciences (IMIC), it was showcased that Togni reagents, when mixed with sodium ascorbate, a cheap, non-toxic and biocompatible reducing agent, immediately generate ?-substituted tetrafluoroethyl radicals that selectively attach to sterically accessible tryptophan residues of the protein under transition metal-free conditions.
Once azidofluoroalkyl groups are attached to the protein, various functional groups, such as fluorescent dyes, radionuclides or ADC-toxins for targeted oncotherapy can be subsequently linked via click reaction to afford the corresponding protein conjugates. The disclosed tryptophan-selective bioconjugation method does not disrupt protein disulfide bridges and offers an alternative solution where conventional cysteine conjugation is not possible, for example, due to an undesirable disulfide scrambling.
Site-selective bioconjugation of tryptophans Credit: IOCB Prague
This bioconjugation method can also be extended to other aromatic amino acids and hence to proteins lacking tryptophan. Thus, it was possible to successfully modify human recombinant insulin and attach up to seven modifications to its aromatic amino acids, demonstrating the potential for post-translational modification of proteins.
The extremely rapid nature of this reaction targeting solvent-accessible aromatic amino acids makes it a convenient tool for mapping protein surfaces and studying protein-protein interactions. Using the example of human carbon anhydrase, the researchers demonstrated that the results of surface mapping are in excellent agreement with its published native structure.
Prof. Dr. Martin Fusek, CEO of IOCB Tech, says, "The basis of successful commercial outcomes from basic research results is excellent scientific work. This is an important result that is not only useful as a tool for basic research but also as a means for the development of new protein-based drugs. The uniqueness, which should rather be the rule, is that the project was created by the cooperation of two academic institutions and one commercial company. I am very glad that we could have been part of the process."
Dr. Petr Beier, head of a research group at IOCB Prague, says, "I am glad that we have been able to develop a successful cross-disciplinary collaboration of organic synthesis and biochemistry. It turns out that the specific properties of fluorinated compounds can be utilized not only traditionally in the medical chemistry of small molecules, but as recently showcased also for bioconjugation of proteins and studies of their structure. I believe that in the future we will be able to identify other attractive uses of Togni reagents in biochemistry."
Dr. Petr Novák, head of a research group at IMIC, says, "Thanks to Togni reagents we have been able to introduce a fluorinated probe into the protein structure in an aqueous environment in a matter of few seconds. We are now able to use this technology to selectively tag proteins for clinical diagnostics or use to it to identify the interaction interface of proteins with their ligands."
Dr. Václav Matou?ek, CEO of CF Plus Chemicals, says, "I am excited to see that the reactivity of Togni reagents could be extended to aromatic amino acids and aromates in general, thus opening a plethora of potential applications, especially in protein science and protein-based therapeutics. We are now actively looking for established industrial partners who could apply our technology to solve their challenges."

