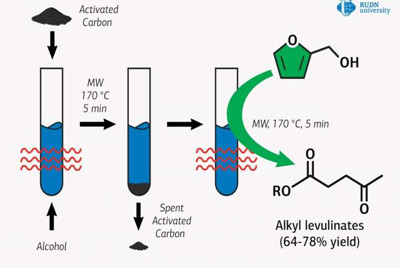
A chemist from RUDN University has found an environmentally friendly way to obtain esters of levulinic acid used as a fuel additive for chemical synthesis. Together with colleagues from France, Greece and Spain, he obtained these esters from furfuryl and aliphatic alcohols and used activated carbon as a catalyst under microwave exposure. The results were published in the journal Molecular Catalysis.
Levulinic acid esters are used as plasticizers. These are substances that are introduced into the composition of polymeric materials in order to confer or increase elasticity or plasticity. The conventional way to obtain these esters, i.e. by direct interaction with alcohols, is considered expensive due to the high cost of the original substance, levulinic acid.
There is a more economical option: to replace it with furfuryl alcohol, which forms during the processing of biomass. But this method requires the use of catalysts containing toxic metals or strong acids which afterwards need to be disposed of in a special way. Professor Rafael Luque of RUDN University and his colleagues proposed to use commercially available and environmentally friendly activated carbon as a catalyst. However, it alone cannot provide sufficient activity, so the chemists combined activated carbon catalysis with microwave activation.
"Microwaves have interesting applications in industry, but are not widely applied in the chemical industry. The problem is associated with the low permeability of the microwaves for which continuous flow processes are starting to be implemented for such applications and needs," says Rafael Luque
Researchers added activated carbon to the reagents and placed the mix in a microwave reactor, where it was constantly stirred. Quantitative analysis of the catalysis products was conducted using gas chromatography, and the obtained substance was determined by gas chromatography-mass spectrometry.
It turned out that under optimal conditions, the yield of methyl ester was 78 percent in five minutes, which is comparable to the use of traditional metal-containing catalysts. Luque and his colleagues also obtained esters with ethyl, n-propyl, isopropyl, and n?butyl alcohols. With these substances, if furfuryl alcohol was used and used up completely, the yield of esters was lower than when methyl alcohol was used. But the chemists suggest that these reactions can also be improved to achieve optimal product yields.
The proposed method opens up the possibility of obtaining valuable organic substances using a "green chemistry" approach from biomass processing products, and has a potential for industrial application because it uses activated carbon as a catalyst, which is inexpensive and commercially available in large volumes.
To better understand the mechanism of reaction of furfuryl alcohol with alcohols in the formation of esters, the chemists tried to detect intermediate compounds by gas chromatography-mass spectrometry. But the results suggest that the reaction mechanism is more complex than anticipated.

