Materials engineering technologies aim to control wettability and liquid repellence of material surfaces for diverse applications in and beyond the field of materials science. In a recent report on Science Advances, Yue Liu and a team of researchers in the departments of Materials Science and Engineering, and Chemistry and Molecular Engineering in China developed a general concept to develop metallic porous surfaces with exceptionally powerful, wettability-switch capabilities. To engineer the new surfaces, they used an extremely simple, one-step electrochemical deposition process. The team enabled the wettability switch and manipulated liquid repellant properties by changing the orientation of dodecyl sulfate ions that were ionically bonded to porous metallic membranes during electrodeposition. The resulting surfaces with adjustable wettability could trap diverse lubricants on demand in the pores to create liquid-infused porous surfaces customized for a variety of liquid-repellant properties. The research team demonstrated the applications of liquid-infused porous membranes for encryption, to control droplet transfer and for water-harvesting. Additionally, the materials scientists coated the silver porous membrane onto a copper mesh to engineer a smart, antifouling liquid gate to allow oil or water to pass through on request.
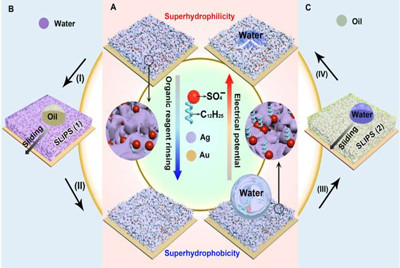
In materials engineering, researchers aim to develop reversibly switchable interfacial properties for diverse applications from microfluidics systems, to water harvesting and transportation, as well as separators, sensors and drug delivery systems. Materials scientists have extensively studied such switchable-wettability surfaces controlled via external stimuli including light, pH value, thermal and electrochemical treatment, counterions and electrical potentials. In the present work, Liu et al. reported an extremely simple, one-step electrodeposition approach to engineer porous metallic surfaces with robust wettability switching capacities coupled to outstanding liquid-repellant properties that differed from previously reported mechanisms to engineer a new wettability switch.
Engineering reversible wettability on electrodeposited porous surfaces
Liu et al. modulated the orientation of dodecyl sulfate ions that ionically bonded to the porous membrane during electrodeposition to establish reversibly tunable surface wettability. For instance, when the dodecyl chains pointed outward, the membrane retained superhydrophobicity (water hating nature). Then the researchers used electric potentials to transition the wettability from superhydrophobic (water hating) to the superhydrophillic (water loving) state. They confirmed ionic surface orientation changes using surface-enhanced Raman scattering (SERS) measurements. Based on the ionic orientation-induced evolution of electrodeposited porous surfaces, the research team firmly trapped diverse lubricants within the pores to form different "slippery liquid-infused porous surfaces (SLIPS)" for multiple applications.
The new technique will have significant potential in diverse thermochemical applications due to its engineering simplicity, versatility and low-cost. To form the reversible wettability switch, the researchers first electrodeposited a silver porous membrane onto a gold-covered silicon wafer in an electrolyte solution containing silver nitrate and sodium dodecyl sulfate (SDS). As the timeline of electrodeposition increased, the membrane roughness gradually increased, after four minutes, the resulting membrane was superhydrophillic. With the assistance of organic liquids as well as with water containing a minute amount of ethanol (one percent in volume), the scientists could induce the transition of wettability from superhydrophillic (or superoleophillic; water loving) to superhydrophobic (water hating). The instant transition indicated high sensitivity of the membrane toward organic reagents. The water contact angle also increased (hydrophobic character) when they simply exposed the silver porous membrane to an ethanol atmosphere (i.e organic reagent).
Understanding the mechanism of wettability transition
The research team conduced systematical studies to reveal the underlying mechanisms of wettability transition, first by using Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) for surface analysis. They proved the existence of SDS ions within the silver porous membrane to form a monolayer structure similar to a previously well-studied surface. Liu et al. hypothesized that hydrophobic dodecyl chain tails hid inside the pores of the freshly prepared silver porous membrane– prompting the silver membrane to initially demonstrate hydrophilicity due to the exposed, hydrophilic sulfate heads.
When the superhydrophillic surface then encountered organic reagents such as ethanol, the hidden dodecyl chain tails changed orientation to face the organic liquids due to their mutually strong affinity. Due to challenges of proving the changing orientation of the dodecyl chains on the rough porous membranes using conventional scanning tunneling microscopy or atomic force microscopy, the team used SERS. The SERS intensity, which mapped the interactions between dodecyl ionic chains and silver sulfate surface, confirmed the transition that facilitated the membrane wettability switch. When they removed the dodecyl ions using oxygen plasma treatment, they eliminated the wettability switch from the silver porous membrane.
Applications of the technique—encryption and liquid transfer
Having developed a new concept to engineer reversible wettability, the research team developed a variety of applications such as information encryption, droplet transfer, liquid-repellence, fog harvesting, and smart liquid gate as well as oil/water separation. For information encryption, Liu et al. dragged a pencil that behaved as a cathode electrode immersed in a droplet, upon the superhydrophobic surface connected to the positive pole of the power supply to write the letters "ZJU." The surface then transformed to maintain hydrophilicity and when the scientists exposed the surface to water or steam, the encrypted invisible words were revealed due to surface attachment of water droplets. They could change the speed of encryption and remove the hydrophilic track using ethanol to recycle the surface multiple times for reuse. By changing surface properties, they could also induce conditions of hydrophobic encryption. The research team then made use of strong surface adhesion to transfer water droplets across from superhydrophillic surfaces using ethanol-treated silver porous membranes, where droplets adhered on top of the silver membrane for easy transfer.
Harnessing the liquid-repellent properties for additional applications
Thereafter, the research team designed silver porous membranes to change from superhydrophillic SLIPS1 (slippery liquid-infused porous surfaces) to superhydrophobic SLIPS2 to form a repeatable cycle between SLIP1 and SLIP2 surfaces for a desired timeframe. The work described here were a first in study to engineer such complex wetting and liquid-repellent properties with potential for dynamic adjustment to match diverse lubricants. Additionally, bioinspired by the Namib desert beetle that used patterned hydrophilic and hydrophobic elytra (hardened forewing) to retain or remove water droplets, Liu et al. patterned stripes of SLIP2 for excellent water repellence. They engineered surfaces to adhere water molecules for nucleation on hydrophilic surfaces upon exposure to water mist for outstanding fog harvesting efficiency.
Next, the scientists electrochemically coated the silver porous membrane onto a copper mesh for applications as a smart liquid gate. While the original superhydrophobicity prohibited the passage of water, when they applied a negative electric potential to the copper mesh, the surfaces became superhydrophilic for the immediate passage of water. The surface property could be interchangeably engineered by exposure to ethanol vapor, for reuse. Liu et al. similarly engineered silver-coated copper meshes for selective water/oil separation, which differed from existing prototypes for efficient oil and water isolation and transfer.
In this way, Yue Liu and colleagues developed a general concept to engineer metallic coatings with switchable wettability liquid repellence using a simple, one-step electrodeposition method. They harnessed the changing orientation of dodecyl sulfate ions ionically bonded to the electrodeposited porous metallic membrane, with organic reagent treatment or an external electric potential to facilitate the wettability switch. They recycled the wettability transition more than 10 times in the study, while forming diverse SLIPS for a variety of applications. The extremely simple and cost-effective materials engineering approach to form switchable wettability and liquid-repellant materials surfaces will have promising applications in liquid/thermal-related fields within and beyond materials science.

