RUDN University chemist Erik Van der Eycken has come up with a new method for the synthesis of a large group of complex poly-heterocyclic organic compounds, which may have applications for new pharmaceuticals. The researcher turned to a two-step reaction in which he used affordable and cheap organic reagents and catalysts based on gold, which allowed him to synthesize as many as 28 new molecules. This approach will significantly expand the libraries of biologically active substances. The work was published in Organic & Biomolecular Chemistry.
The search for new medications usually starts not with doctors, but with synthetic chemists. They seek to develop so-called libraries—a number of similar organic substances that can potentially exhibit biological activity. Dealing not with "individual" substances, but with libraries makes it easier to isolate highly effective compounds that may be slightly different from their less effective analogues, "neighbours" in the library.
"Diverse small molecular libraries constitute a significant part in the drug discovery process. In recent decades, the development of high-throughput screening has enabled the extremely rapid biological evaluation of large collections of small organic molecules. However, growing evidence now suggests that many existing compound collections are inadequate in the search for new molecular entities capable of interacting with complex biological targets. Thus, generating diverse molecular libraries is still in high demand," says Van der Eycken.
There are difficulties with creating some such libraries, e.g., for polycyclic molecules with several heteroatoms—nitrogen, oxygen, or sulphur. Most of the known compounds from this group exhibit biological activity. For example, the group includes vincamine, a drug for stimulating cerebral circulation in dementia, or tsitsikammamin, a topoisomerase inhibitor, known for its antimicrobial and antimalarial effect. However, there is no universal method for obtaining these compounds, and in order to verify their pharmacological action, it is necessary to come up with a particular and often multi-stage synthesis scheme for each of them.
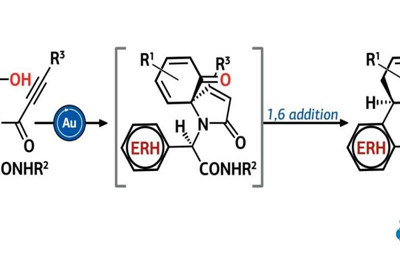
Van der Eycken came up with a method that obtains compounds from this group using only widespread and cheap reagents. To achieve this, the chemist chose the multicomponent Ugi reaction for the first stage of the scheme. This reaction combines four simple organic molecules into a single structure. It helped Van der Eycken to obtain an aromatic polycyclic molecule with several heteroatoms out of indole, pyrrole, benzothiophene or furan.
The next stage in the proposed scheme was the dearomatisation reaction, that is, the saturation of double bonds in the benzene ring combined with spirocyclisation, i.e., the formation of ring structures inside the molecule in which one carbon atom belongs to several cycles at once. Van der Eycken used this main stage for such compounds for the first time.
He managed to select the catalyst and the temperature under which aromaticity is broken in aromatic heterocyclic structures, and sites from the opposite end of the complex molecule are attached to several carbon atoms. In total, the chemist checked 15 catalysts with similar composition based on gold chloride, and one of them made possible a yield of 53 to 98 percent, depending on the structure of reacting substances. The optimal synthesis temperature was from 70 to 110 degrees, and the synthesis time was from two to 30 hours.
The proposed scheme is best used for obtaining compounds in which the rings either do not contain side groups at all, or in which such groups have an aromatic structure. But since the acetyl side group generally stops the reaction, it can be used as a protective element in such a scheme. In total, the chemist synthesized 28 compounds, each of which has the necessary polycyclic structure, which significantly expands the library of candidates for medications and biologically active substances.
"Our newly developed protocol enables the rapid construction of diversity-oriented complex natural product-like poly-heterocyclic compound libraries in a high atom- and step-economic manner, which appears as an emerging tool for high-throughput screening in drug/lead discovery. The generated complex small molecular libraries will be used for high-throughput screening," Erik Van der Eycken says.

